
Application notes
Effect of temperature on sulfur powder flowability
In this work, we show how the powder form, composite or dry-mixed, of the sulfur/carbon blend influences the flowability of this powder and the dependency of this flowability with temperature, using the GranuDrum High Temperature and the GranuPack instruments.

![]()
![]()
Introduction
Lithium-sulfur batteries are seen as good alternatives to replace Lithium-ion batteries that dominate the current market. The theoretical high gravimetric capacity of sulfur and its cheap price are good arguments in favor of sulfur as an active material. Nevertheless, sulfur has the drawback of being a poor conductor. For that reason, a large quantity of carbon black has to be added to obtain sufficient conductivity. This reduces considerably the quantity of sulfur that can be put in the battery. Composite powders of sulfur and carbon are seen as a solution to this problem, combining the high energy density of the sulfur with the good conductivity of the carbon black in a single material. In such a form, the grains are an amalgam of sulfur and carbon as shown in Figure 1.
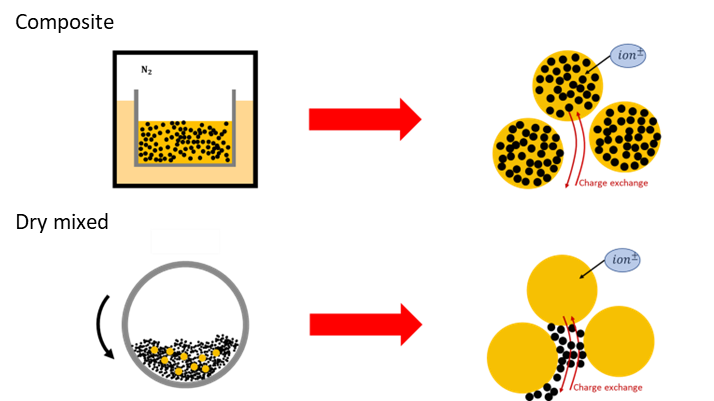
Figure 1: Representation of composite or dry mixed form.
Such materials are however challenging to handle for electrode production, especially with the upcoming dry processes. Indeed, in a recent study [1], it has been proved that a binder-free dry process is possible to produce a cathode made of sulfur and carbon. A composite of sulfur and carbon in powder form was spread into a squared frame and then compressed under a hot press. This opens new perspectives for lithium-sulfur batteries production but also brings new challenges. Indeed, such an innovative process may require optimization to reduce cost and improve electrode quality. The flowability of the powder material is doubtless an essential parameter to improve since the powder needs to be spread in a thin and homogeneous layer before hot pressing. Furthermore, the dependency of the flowability with temperature is of huge importance.
In this work, we show how the powder form, composite or dry-mixed, of the sulfur/carbon blend influences the flowability of this powder and the dependency of this flowability with temperature. The investigation was conducted with the GranuPack and GranuDrum High Temperature.
Material and method
Two powder samples made of sulfur and carbon were tested. For both, a proportion of 10 units of sulfur for 1 unit of carbon in weight was used. The first batch was prepared by dry mixing the sulfur with the carbon black powder. The second, the composite form, was done by melting the sulfur and introducing it into the porous matrix of the carbon black powder. To do so the sulfur and the carbon black are put together in a container under a nitrogen atmosphere and heated with an oil bath of around 130-140°C to melt the sulfur. Once the sulfur is in liquid form, it is blended with carbon black to homogenize the mixture and to fill the porous medium made by the carbon black powder. Then the mixture is cooled and milled to obtain a powder. The milling was performed to obtain a size distribution equivalent to the dry-mixed batch with D50 around 50µm.
The powders were characterized with the GranuPack High Temperature and the GranuDrum High Temperature to assess the effect of temperature on their flowability.

Figure 2: (left) Picture of a GranuPack High Temperature. (right) Picutre of a GranuDrum High Temperature.
Both instruments work identically to their equivalent without temperature control. The difference is the addition in the protocol of a preheating procedure. Once the powder is filled in the cell of the GranuPack or GranuDrum High Temperature, the instrument heats the cell up to the target temperature (from room to 200°C for the GranuPack and from room to 250°C for the GranuDrum). When the temperature is reached, the instrument waits for a specified time, to let the powder temperature be homogenized. Then the measurement starts. During the measurement, the temperature is kept constant, monitored, and recorded in an Excel file.
In this work, the powders were tested with both instruments with identical measurement parameters at different temperatures from room to 100°C. The waiting time was set at 30 min for the GranuPack High Temperature and at 10 min for the GranuDrum High Temperature to ensure a homogeneous heating of the powders. The number of taps was set at 500 for the GranuPack and an increasing speed sequence (2;4;6;8;10;12;14;16;18;20;25;30;35;40;45;50;55;60) was used for the GranuDrum.
LEARN MORE ABOUT OUR INSTRUMENTS
Results and discussion
In Figure 3, the packing curves of the composite and the dry mixed powders at different temperatures are presented. Table 1 resumes the metrics obtained from these packing curves. One notices that a large effect of the temperature on the packing is observed for the composite powder while few differences are seen for the dry mixed powder with temperature.
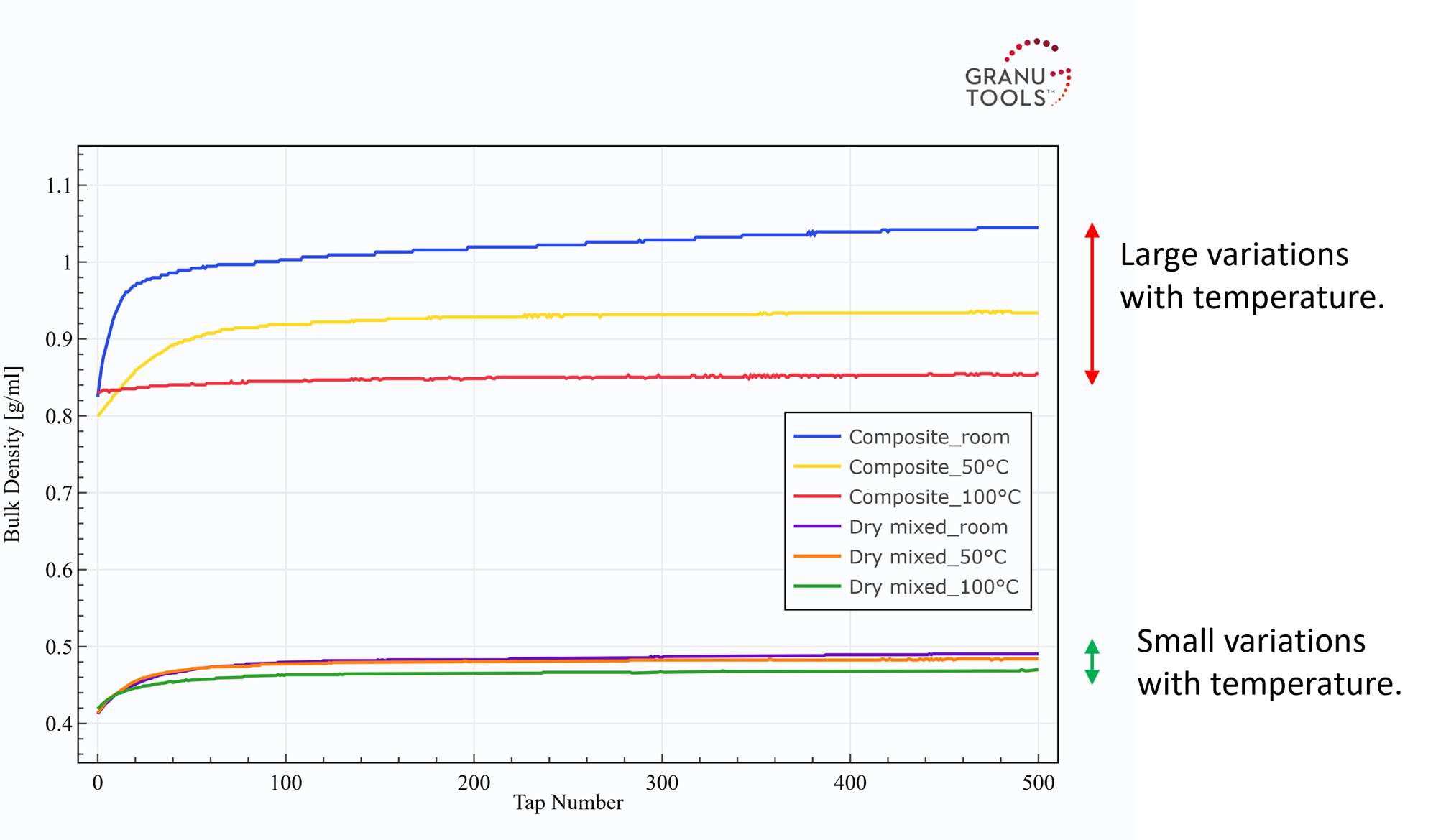
Figure 3: Effect of temperature on the packing curves of the composite and the dry mixed powders.
| Name | ρ(0) [g/ml] |
ρ(500) [g/ml] |
Hr | α [g/l] |
n1/2 |
| Composite room | 0.825 | 1.045 | 1.27 | 11.3 | 10.0 |
| Composite 50°C | 0.799 | 0.934 | 1.17 | 3.1 | 25.0 |
| Composite 100°C | 0.829 | 0.855 | 1.03 | 0.4 | 50.0 |
| Dry mixed room | 0.412 | 0.490 | 1.19 | 2.6 | 21.0 |
| Dry mixed 50°C | 0.414 | 0.484 | 1.17 | 2.7 | 16.0 |
| Dry mixed 100°C | 0.419 | 0.470 | 1.12 | 1.9 | 18.0 |
Table 1: Bulk densities (initial and final), Hausner ratio, and the initial packing speed obtained from the experiments with the GranuPack High Temperature.
For the composite powder, the packing dynamic, assessed by α decreases. This may be explained by partial melting or sintering between particles that contain sulfur (the melting point is between 130-140°C) which creates solid bridges between these particles. As a consequence, the sintered particle cannot move to reorganize the packing and increase the density which results in a decrease in the initial densification speed α. In parallel, n1/2 increases accordingly as α decreases. An effect of the decrease in packing dynamic is the reduction of the reachable final bulk density ρ(500). Therefore, it is normal that the Hausner ratio decreases with temperature. This phenomenon is generally observed when playing with temperature on plastic or food powders (more information can be found in [2,3]). On the contrary, Little changes are obtained by increasing the temperature with the dry mixed powder.
Similar observations are seen with the GranuDrum HT experiments. Indeed, in Figure 4, no clear difference is seen between the curves of Cohesive Index at different temperatures for the dry mixed powder. This means that the dry mixed powder has the same flowability at room or hot temperatures (up to 100°C). Therefore, the processability of this powder is expected to be unchanged if the powder is handled at room or at 100°C in a dry process for electrode manufacturing. However, we point out that this powder improves its flowability when the undergone shear rate increases since a decrease in cohesion is obtained with an increase in the rotating speed. For the composite powder, the flow was only possible at room temperature. Even at 50°C, the flowability was so low, due to the probable sintering of particles, that the powder formed a soft material but poorly deformable.
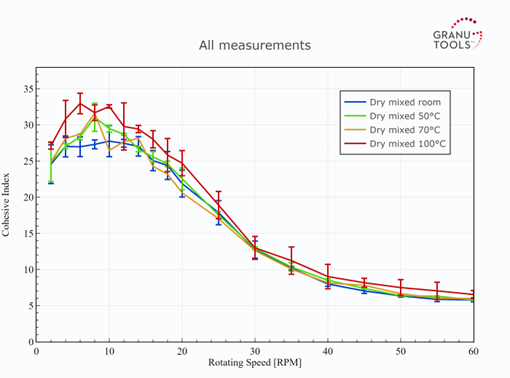
Figure 4: Effect of temperature on the flowability of the dry mixed powder.
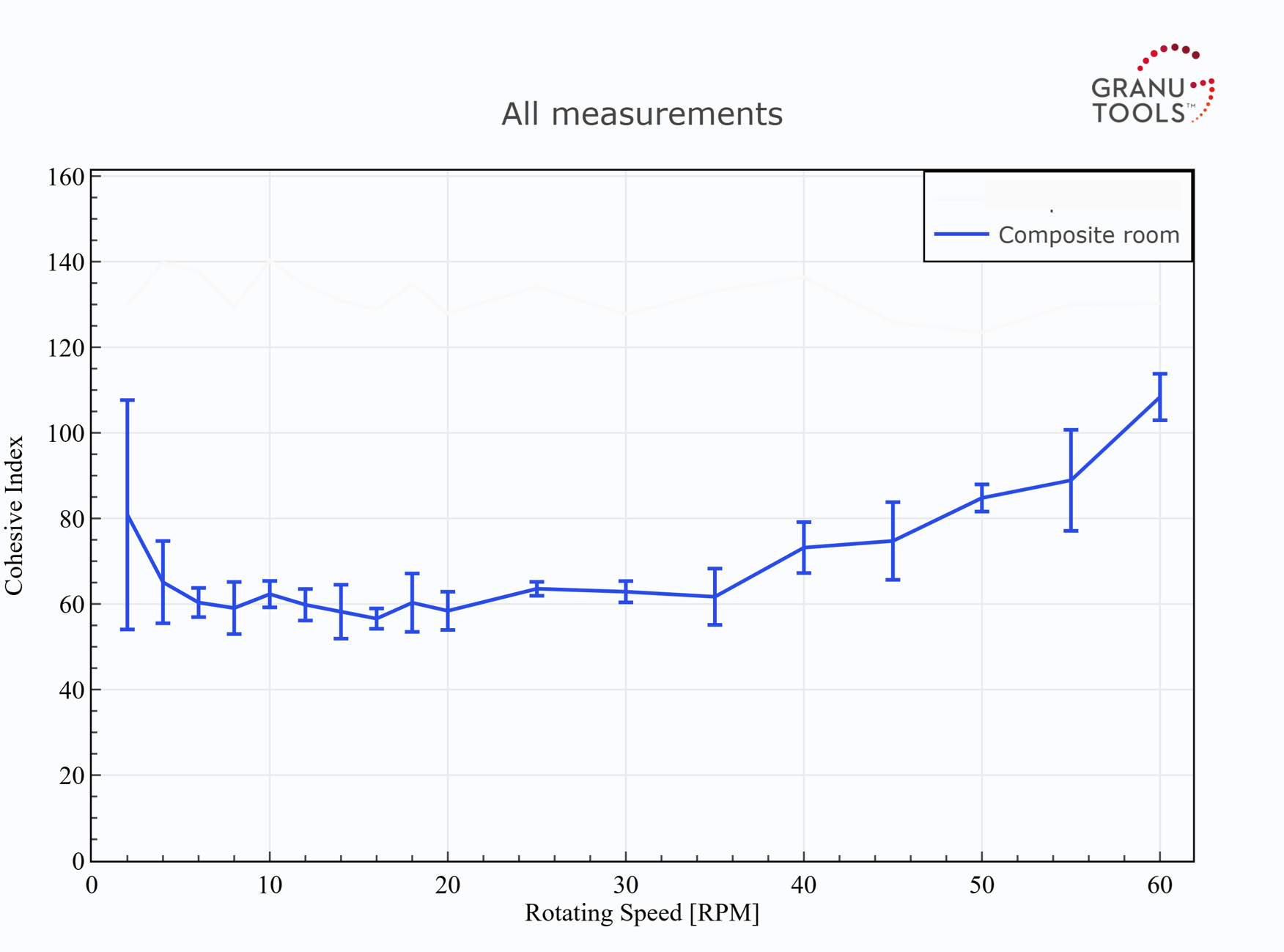
Figure 5: Effect of temperature on the flowability of the composite powder.
The combination of the GranuPack and GranuDrum HT measurements brings out that the flowability and therefore the processability of the composite powder is more sensitive to temperature change than the dry mixed powder. This has consequences for processes that handle powder in hot conditions. This could fit into the context of electrode production in which powders are more and more handled in dry and hot conditions.
In addition, from a more general perspective, it is also interesting to observe that even having the same proportion of chemical materials, two powders having a difference in the structure of the particles leads to different sensitivity to temperature. This means that the chemical nature of the particles and the structure that they form inside the powder matter for the dependency with temperature, highlighting the importance of characterizing this dependency. Here, it is possible that for the dry mixed powder, the carbon black powder forms a thin coating of carbon, limiting the formation of a solid bridge of sulfur between particles which is not the case of the composite powder. This may explain why the flowability of the dry mixed powder does not change with temperature increase while the flowability of the composite powder does. Nevertheless, this needs a deeper investigation to attest it.
Comparing now the flowability of the dry mixed powder with the composite powder, it is clear that the dry mixed powder has a better flowability than the composite powder at room and high temperatures. Indeed, the dry mixed powder exhibits a lower Cohesive Index and a lower Hausner ratio than the composite form at room temperature. At high temperatures, the composite powder is simply unable to flow in the GranuDrum, attesting to a very poor flowability while the dry mixed powder keeps a good flowability. Furthermore, the dry mixed powder exhibits a decrease in cohesion with an increase in rotating speed in the drum (shear-thinning behaviours). This means that this powder decreases its cohesion and thus increases its flowability as the undergone shear rate increases. This is generally an advantageous effect in a manufacturing process. Indeed, the speed of the process can be increased with an improvement in powder flowability as a consequence. On the contrary, the composite powder exhibits an opposite behaviour (shear-thickening), being generally detrimental to manufacturing processes.
Conclusion
Dry powder handling is more and more a concern in electrode production, especially for dry processes that directly transform the battery material initially in powder form into a free standing film. This film is then coated on a metal foil to produce the electrode. To obtain good properties for this film, it is important that the powder can be handled adequately in the process. Therefore, the powder must have good flowability. Moreover, it is important to know how this flowability depends on temperature since electrode manufacturing implies more and more elevated temperatures for the dry processes. Therefore it is important to quantify the flowability of such powders and their dependency to temperature for electrode manufacturing improvement.
Here two powder blends of carbon black and sulfur were tested with the Granupack and the GranuDrum High Temperature to asses their flowability and the effect of temperature on it. One was prepared by dry mixing the carbon black with the sulfur. The other one was a composite of the two chemical compounds. The aim was to compare the flowability of composite powder with dry mixed powder and how it evolves with temperature. The composite powder of sulfur is generally seen as a good solution for improving conductivity and specific capacity, while the dry mixed powder is more conventional, with less performance in terms of energy storage.
From the results of the GranuPack and the GranuDrum High Temperature, it appears that the dry mixed powder has better flowability and a lower dependency to temperature compared to composite powder. Therefore, while the composite powder has better performances for energy storage and conductivity compared to the dry mixed powder, its handling is more challenging. Consequently, there is a compromise between the performance of the powder and its processability between the dry mixed and the composite powders. This can be solved by a characterization of the flowability of the different powders, according to the different external factors such as the shear rate and the temperature undergone in the process, and comparing it to the cost and the efficiency of the produced battery. The GranuPack and the GranuDrum High Temperature are adequate tools for such investigation.
Bibliography
[1] M. Horst, J. K. Burmeister, M. Abdollahifar, S. Pillitteri and A. Kwade, A binder-free dry coating process for high sulfur loading cathodes of Li–S batteries: A proof-of-concept, J. Power Sources 587, 233675 (2023).
[2] G. Lumay, F. Francqui, C. Detrembleur and N. Vandewalle, Influence of temperature on the packing dynamics of polymer powders. Adv. Powder Technol. 31, 4428-4435 (2020).
[3] A. Neveu, F. Francqui and G. Lumay, Packing dynamics of powders at high temperature. In EPJ Web of Conferences, EDP Sciences 249, 12001 (2021).
[5] H. Bockholt, M. Indrikova, A. Netz, F. Golks and A. Kwade, The interaction of consecutive process steps in the manufacturing of lithium-ion battery electrodes with regard to structural and electrochemical properties, J. Power Sources 325, 140-151 (2016).
[6] H. Bockholt, W. Haselrieder and A. Kwade, Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes, Powder Technol. 297, 266-274 (2016).
[7] Y. J. Nam, D. Y. Oh, S. H. Jung and Y. S. Jung,Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry-and slurry-mixing processes, J. Power Sources 375, 93-101 (2018).
[8] A. Diener, S. Ivanov, W. Haselrieder and A. Kwade, Evaluation of Deformation Behavior and Fast Elastic Recovery of Lithium‐Ion Battery Cathodes via Direct Roll‐Gap Detection During Calendering, Energy Technol. 10.4, 2101033 (2022).
