Application notes
Bulk powder characterization to optimise battery manufacturing and performance
In this study, we highlight the advantages provided by producing composite powders made of sulfur and carbon black compared to the simple dry-mixed powder blend method for Sulfur Li-ion based batteries.



Introduction
Generalities
L-ion batteries are the dominant part of battery manufacturing and market due to their good performance in terms of energy and power densities. However, the shortage of rare-earth elements and the need for new-generation batteries lead to a focus on different types of batteries. A new alternative envisaged to reduce the cost and the environmental impact of battery manufacturing is lithium-sulfur (Li-S) batteries, in which sulfur replaces the common Li-oxides as active cathode material The theoretical gravimetric capacity of Li-S batteries significantly surpasses the specific capacity of state-of-the-art lithium-ion batteries, which is promising for applications where the weight of the battery is a limiting factor, such as electric cars, drones etc. Sulfur, in addition, is relatively cheap, and abundant compared to the transition metals (e.g., Co, Ni) used in conventional lithium-ion batteries. However, Sulfur has a poor conductivity, which is a drawback for battery application. Therefore, carbon black is used as the conductive additive resulting in a low volumetric energy density due to this crucial quantity of conductive additive. Therefore, the density of the active material must be improved to compensate for this problem. Moreover, since raw materials for battery manufacturing are handled in powder form at the beginning of the process, the bulk properties of sulfur in powder form are important to ensure a good flowability and easy processability to facilitate its handling.
The manufacture of an electrode consists of three important steps: the powder blend preparation, made of active material, conductive additive and binder, all three in powder form, the spread of this mixture in the powder form (dry process) or a slurry form (with added solvent) on a current conductor sheet, and the packing of these produced electrodes in a cell usable in a battery. Since electrode manufacturing is a crucial step for battery quality, a good flowability is needed for easy handling during conveying or spreading during dry processes. For these reasons, specific care is paid for the improvement of the raw powder properties of sulfur to meet the quality criteria.
Scope of the study
While sulfur is far cheaper than multi-metal lithium oxides for electrode manufacturing, it is necessary to improve its characteristics to make it usable for battery production. While mixing raw sulfur with conductive additives and other excipients can help to improve these properties, it is nevertheless insufficient. To overcome this drawback, composite powders are envisaged. It involves the melting of sulfur in a porous matrix of a conductive excipient, generally carbon black, to simultaneously increase the density and reduce the resistivity of the powder blend.
In this application note, we discuss the impact of the powder preparation by comparing the bulk properties of blends made of sulfur and carbon black prepared by infusion of the sulfur to make a composite powder with blends prepared by dry-mixing as reference powders. The packing and cohesiveness analysis were respectively done with the GranuPack and the GranuDrum.
Powders characteristics and preparation
Material and methods
Due to its poor electronic conductivity sulfur powder is often mixed with carbon black before being processed to produce electrodes. In this study, we decided to compare two methods of mixing for active material preparation. The first consist of dry-mixing the sulfur powder with the carbon black powder as illustrated at the top of Figure 1. In this study, we named ‘’reference powders’’ the powders produced by this ‘’conventional’’ method. The second method consists of melting the sulfur and mixing it with the porous medium made by the carbon black as illustrated at the bottom of Figure 1. The procedure is done as follows: the sulfur and the carbon black are put together in a container under a nitrogen atmosphere and heated with an oil bath of around 130-140°C to melt the sulfur. Once the sulfur is in liquid form, it is blended with carbon black to homogenize the mixture and to fill the porous medium made by the carbon black powder. Then the mixture is cooled and milled to obtain a powder. Thereby, composite particles of sulfur and carbon are created and we named ‘’composite powders’’ the powders made by this method.
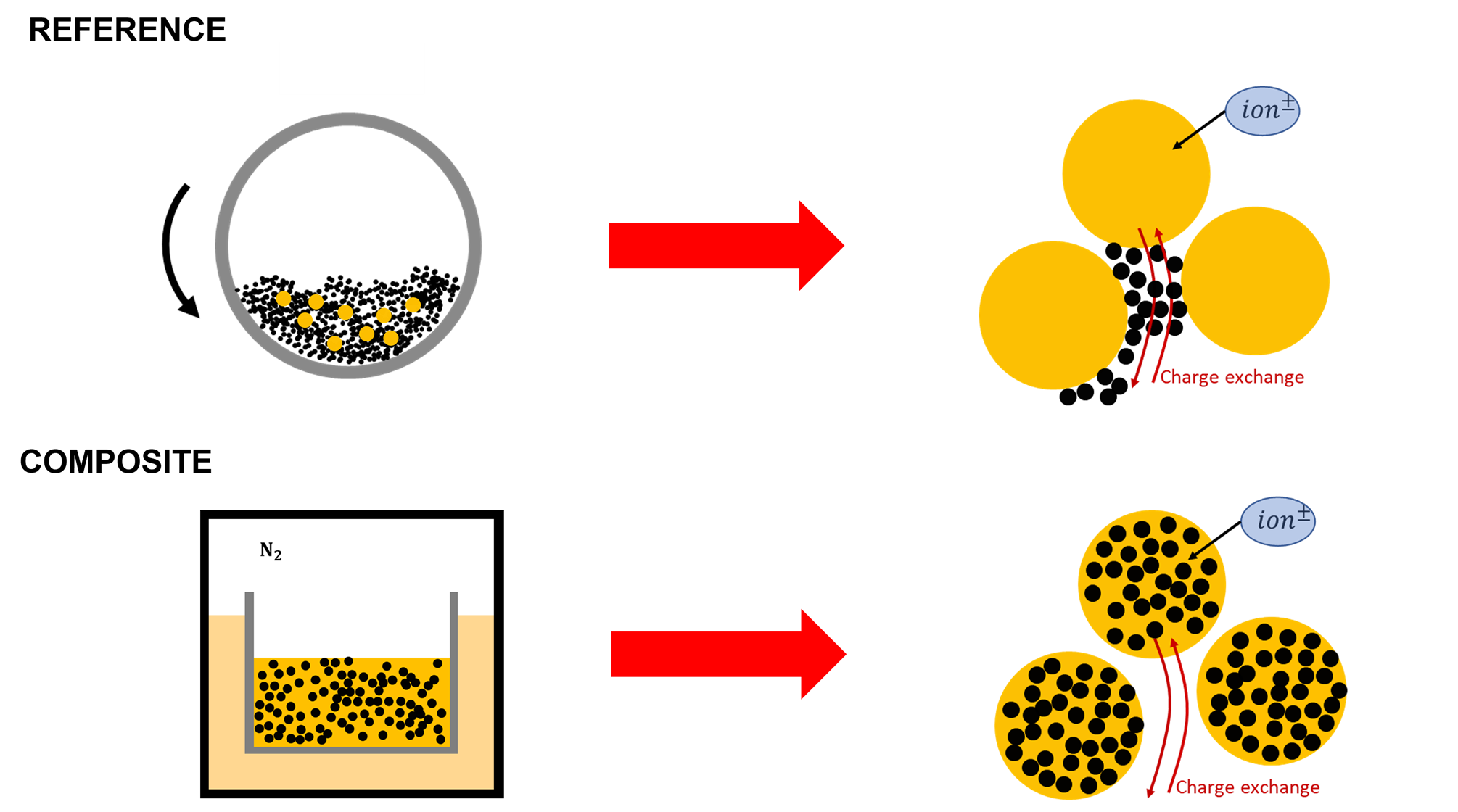
Figure 1: Sketch of the two different methods of preparation
Materials proportions
In this study, two proportions of sulfur and carbon were investigated: 10 units in weight of sulfur for 1 unit in weight of carbon, referenced as S10C1, and 10 units of sulfur for 2 units of carbon in weight, referenced as S10C2. In this way, the effect of the carbon rate in the sulfur was evaluated and each composite powder was compared with its reference equivalent in terms of chemical composition. Four powder configurations were therefore characterized in this work: composite S10C1, composite S10C2, reference S10C1 and reference S10C2.
For comparing batteries with equivalent chemical compositions, the electrodes were produced from fixed proportions of raw materials: 66% sulfur as the active material, 24% carbon black as the conductive additive, and 10% LiPAA(Lithium Polyacrylate) as the binder. It means that composite and reference powders were made with all the sulfur and only a fraction of all carbon black. The binder and the rest of carbon black were only added for the electrodes production. Solvent has been added to the raw materials to make a slurry which was spread and dried to create an electrode.
Therefore, the composite S10C1 represents 72.6% (66% sulfur + 6.6% carbon) of the total electrode material. For the manufacturing process, this composite powder is mixed with the rest of the carbon black that was not used for the composite, representing 17.4% of ‘’pure’’ carbon black in powder form. The S10C2 composite represents 79.2% (66% sulfur + 13.2% carbon) of the total material and the rest of ‘’pure’’ carbon black represents 10.8%.
Packing properties analysis
Experimental protocol
The packing density of each powder was characterized with the tapped density method. This method, also named “tap-tap test”, is popular for its simplicity and its rapidity. It exists standardized procedures with specific protocols to perform this kind of measurement. However, most standardized methods have major drawbacks. The filling procedure is done by the operator which influences the initial powder volume, and the tapped volume is measured by naked eye.
In this work, we used the GranuPack high resolution tapped density analyzer (see Figure 2) which removes these drawbacks with a fully automated protocol for the filling and the measurement of the powder volume. The powder is poured in a metallic tube with a rigorous automated initialization protocol. Afterwards, a light hollow cylinder is placed on the top of the powder bed to keep the powder/air interface flat during the compaction process as illustrated in Figure 2. The tube containing the powder sample rose up to a fixed height of ΔZ and undergoes free fall. The height h of the powder bed is measured automatically after each tap. From the height h, the volume V of the pile is computed. As the powder mass m is known, the density ρ is evaluated and plotted after each tap. The density is the ratio between the mass m and the powder bed volume V. The Hausner ratio Hr is related to the powder cohesion and is calculated by the equation Hr = ρ(1500) / ρ(0), where ρ(0) is the initial bulk density and ρ(1500) the tapped density obtained after 1500 taps. From the full packing curve provided by the GranuPack, dynamical parameters characterizing the kinetics of the packing are defined: the number of taps N1/2 needed to reach one half of the packing amplitude and the initial slope α at the beginning of the process. The initial slope α is computed with a linear fitting ρ(n) = ρ(0) + α.n on the initial linear part of the packing curve. The higher the initial slope the faster the packing. This dynamical parameter better describes the behavior at the initial stage of the packing for which the grains benefit from the highest mobility to rearrange.
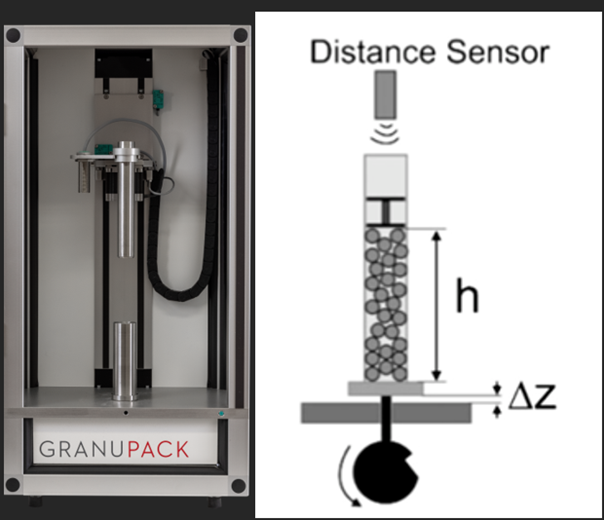
Figure 2: (left) Picture of the GranuPack and (right) sketch of the GranuPack
For each experiment with the GranuPack, 1500 taps were applied to the sample with a tap frequency of 1Hz and 1 mm of free-fall. Measurements were repeated three times for evaluating reproducibility and the average value and standard deviation were considered.
Experimental results
Figure 3 presents the bulk density as a function of the number of taps for the four powder samples. composite S10C1 and composite S10C2 curves have a larger bulk density than reference S10C1 and reference S10C2. A large improvement in density is thus observed for the composite powders compared to the reference powders. Moreover, the composite S10C1 shows a higher density than composite S10C2. The same effect of the relative proportion on the bulk density is observed with the reference powders. However, the initial densities ρ(0) of composite S10C1 and composite S10C2 are almost identical while final density ρ(1500) is completely different.
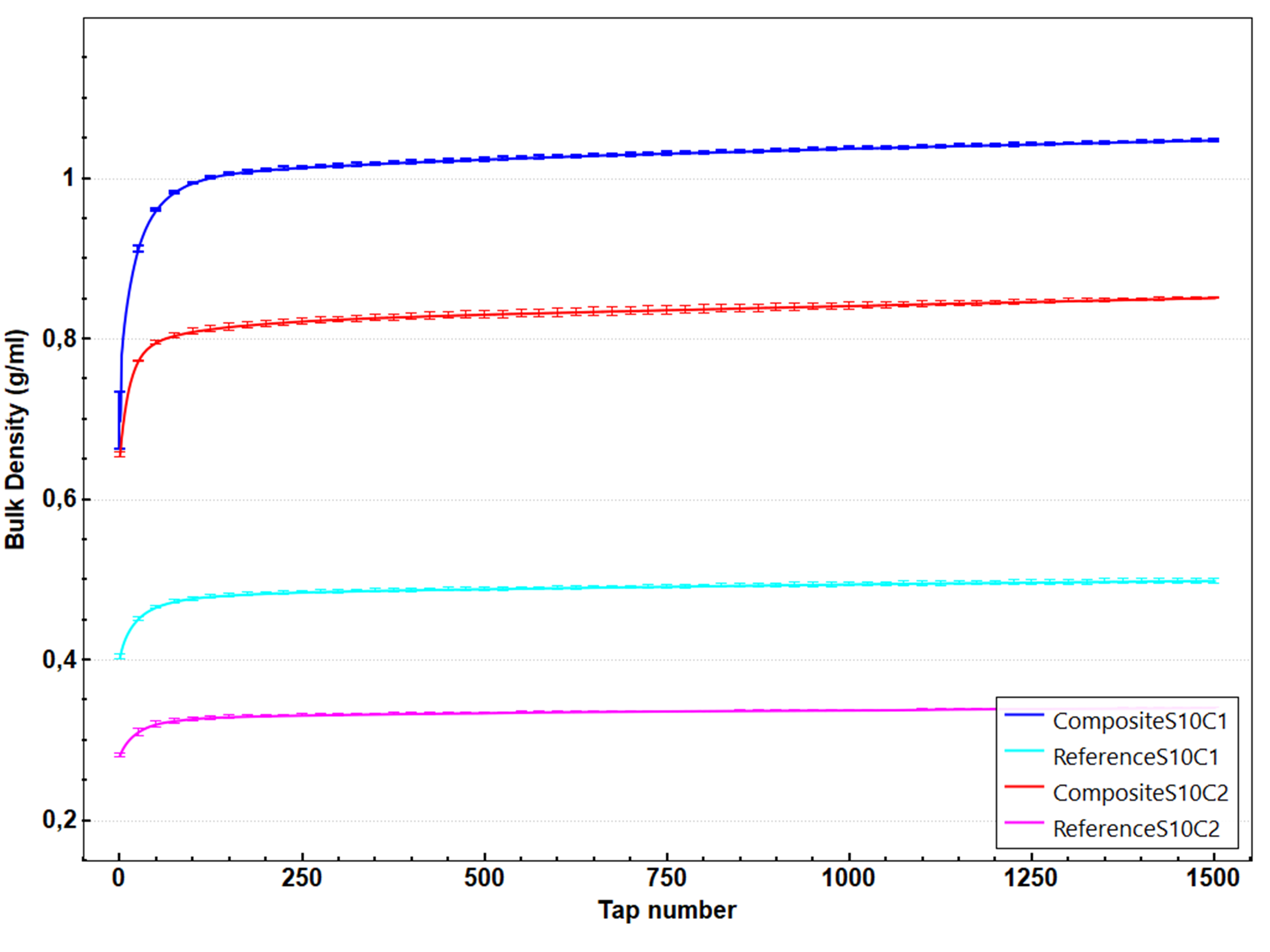
Figure 3: Bulk density variations versus tap number for all powders
The numerical values for the initial and final densities ρ(0) and ρ(1500), the Hausner ratio and the dynamical parameters are presented in table 1. While the composite powders are the densest, they are observed to have a larger Hausner ratio than the reference powders. Moreover, the composite S10C1 which has the largest density shows the largest Hausner ratio. These results suggest that composite powders are more cohesive than dry-mixed powders and the composite S10C1 is assumed to have the largest cohesiveness, often associated with a poor flowability.
The dynamical parameters seem to be well correlated with the Hausner ratio. The slope index α is related to the particle mobility at the initial compaction stage. The larger this mobility, the faster the density increases which gives a large slope index α. For these powders, a large α corresponds to a large Hausner ratio, related to a large cohesion, and decreasing Hr decreases α. composite S10C1 has thus the largest α, followed by composite S10C2. The lowest α is obtained for reference S10C2, following the classification of the Hausner ratio. The large cohesion of composite powders compared to reference ones may allow the powder to sustain a very loose initial packing at rest. It gives room to quickly increase the bulk density after only a few taps, leading to a large slope index α. The second dynamical parameter N1/2, also related to particle mobility, is consistent with α. The lower N1/2, the larger the particle mobility because it takes less time for the powder to densify and to reorganize the packing when the grains are mobile. An increase of α is observed and at the same time a decrease of N1/2 between reference and composite powders. These different dynamics between composite and reference powders could be linked to different particle properties. Indeed, the composite particles are completely different from the particles constituting mixed raw sulfur and raw carbon black powders, influencing the packing mechanisms. The different packing dynamics between composite and reference powders are therefore highlighted with the GranuPack.
| Name |
ρ(0) (g/ml) |
ρ(n) (g/ml) |
Hr |
α (g/l) |
N1/2 |
|---|---|---|---|---|---|
| CompositeS10C1 | 0,698 | 1,047 | 1,503 | 14,75 | 15,67 |
| CompositeS10C2 | 0,656 | 0,851 | 1,297 | 7,40 | 17,33 |
| ReferenceS10C1 | 0,404 | 0,499 | 1,234 | 2,84 | 26,00 |
| ReferenceS10C2 | 0,281 | 0,341 | 1,211 | 1,65 | 27,33 |
Table 1: Results obtained with the GranuPack for the four powder samples
LEARN MORE ABOUT THE GRANUPACK
Powder rheology
Experimental protocol
The powder rheology was investigated with the GranuDrum. It is an automated powder flowability measurement instrument based on the rotating drum principle. A horizontal cylinder with transparent sidewalls is half filled with the sample of powder. The drum rotates around its axis at an angular velocity ranging from 2 rpm to 60 rpm. A CCD camera takes snapshots (30 to 100 images separated by 1s) for each angular velocity. The air/powder interface is detected on each snapshot with an edge detection algorithm. Afterward, the average interface position and the fluctuations around this average position are computed. Then, for each rotating speed, the flowing angle (also known in the literature as ‘dynamic angle of repose’) αf is computed from the average interface position and the dynamic cohesive index σf is measured from the interface fluctuations. In general, a low value of the flowing angle αf corresponds to a good flowability. The flowing angle is influenced by a wide set of parameters: the friction between the grains, the shape of the grains, the cohesive forces (van der Waals, electrostatic and capillary forces) between the grains. The dynamic cohesive index σf is only related to the cohesive forces between the grains. A cohesive powder leads to an intermittent flow while a non-cohesive powder leads to a regular flow. Therefore, a dynamic cohesive index close to zero corresponds to a non-cohesive powder. When the powder cohesiveness increases, the cohesive index increases accordingly.
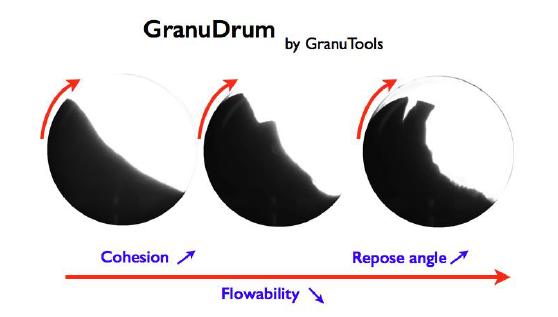
Figure 4: Sketch of GranuDrum measurement principle
In addition to the measurement of both the cohesive index σf and the flowing angle αf as a function of the rotating speed, the GranuDrum allows measuring the first avalanche angle and the powder aeration during the flow.
For each experiment with the GranuDrum, an increasing speed sequence was used from 2 rpm to 60 rpm and the Dynamic Cohesive Index was measured. Measurements were repeated three times for evaluating reproducibility and the average value and standard deviation were considered.
LEARN MORE ABOUT THE GRANUDRUM
Experimental results
Figure 5 presents the Dynamic Cohesive Index as a function of the rotating drum speed for each powder. The curves of composite powders are globally higher than for reference powders that are both collapsed. It means that composite powders have globally a larger Dynamic Cohesive Index, related to a larger cohesion, than dry-mixed powder. composite S10C1 corresponds to the most cohesive powder, followed by composite S10C2 and then reference powders. At low rotating speed, the classification according to the Dynamic Cohesive Index is in good agreement with the Hausner ratio characterization obtained with the GranuPack. The higher cohesiveness of composite powders compared to reference powders is therefore confirmed by both the GranuDrum and the GranuPack measurements.
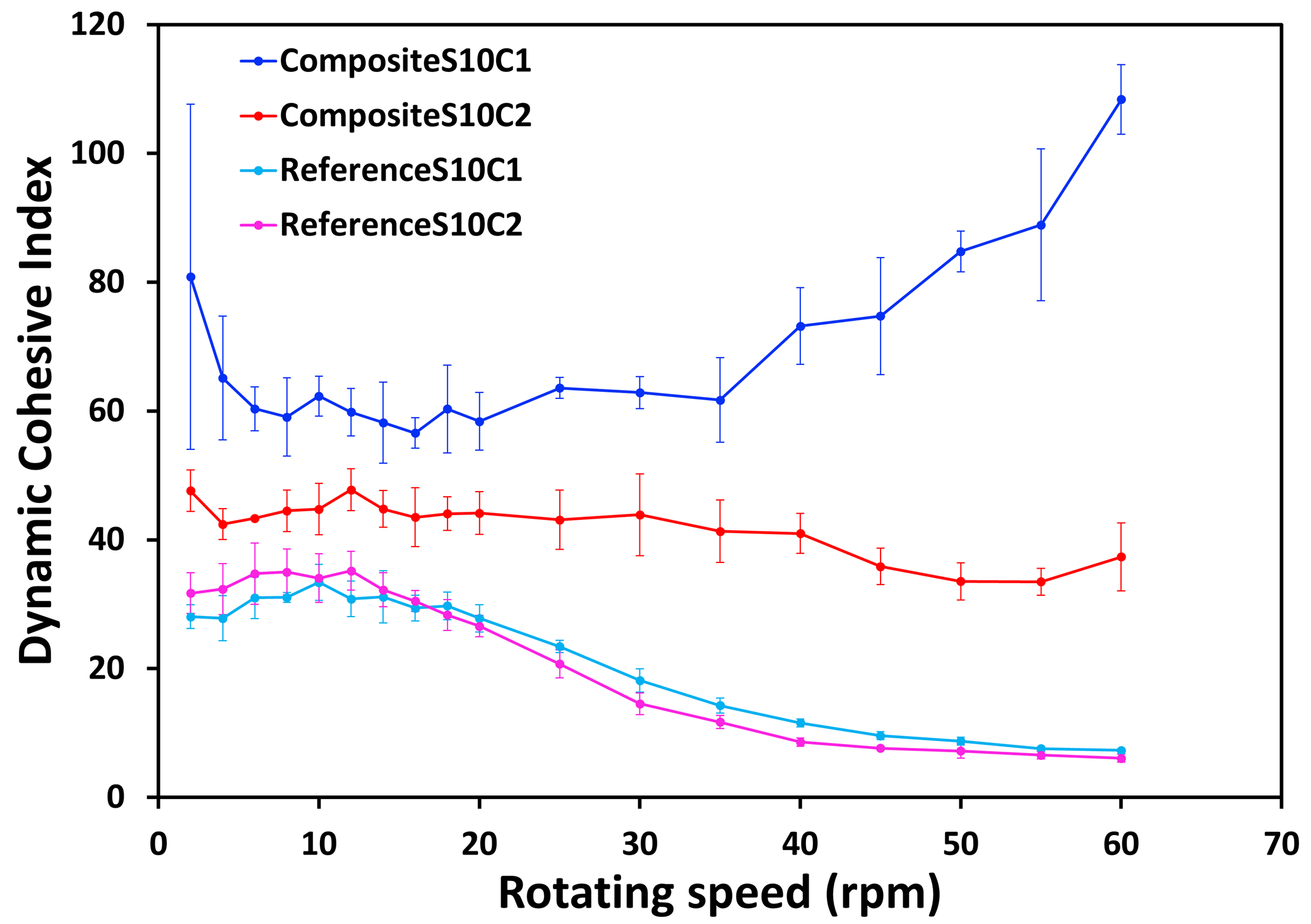
Figure 5: Dynamic Cohesive Index as a function of the rotating speed for all powders
In addition, different rheological behaviours for the four powder samples are measured with GranuDrum. Both dry-mixed powders exhibit shear-thinning, composite S10C1shows shear-thickening and composite S10C2 has a stable cohesion compared to other samples. The shear thinning observed for reference powders means that the cohesion of dry-mixed powders tends to decrease when the applied stress increases. In this case, these powders are expected to better flow during a conveying or a dry-spreading process when the speed or the applied stress undergone by the process increases. On the contrary, composite S10C1 increases its cohesiveness with the applied stress, probably leading to a lower flowability during a dry manufacturing process.
Battery charge/discharge
Experimental protocol
The performance analysis of the tested powders was conducted on coin-type cells. The composite and reference powders were mixed with the rest of carbon black and binder powder to produce electrodes used for the manufacture of batteries. The preparations were mixed with water to form a slurry that was spread on a current collector by doctor-blading and dried. The electrodes were produced with a fixed volume and their surface density was measured. Then, they were packed in batteries and charge/discharge cycles were performed at C/10 or C/40 charging/discharging rates (meaning a complete charge or discharge in respectively 10 or 40 hours) to evaluate the performance of the batteries.
Experimental results
Figures 6 presents the specific capacity at C/10 rate as a function of the number of charging-discharging cycles for the battery produced with reference S10C1 and composite S10C1. Figure 7 presents equivalent results for C/40. In both figures, the battery made with the composite powder shows consistently higher capacity than the battery made with the reference powder, meaning a higher energy density.
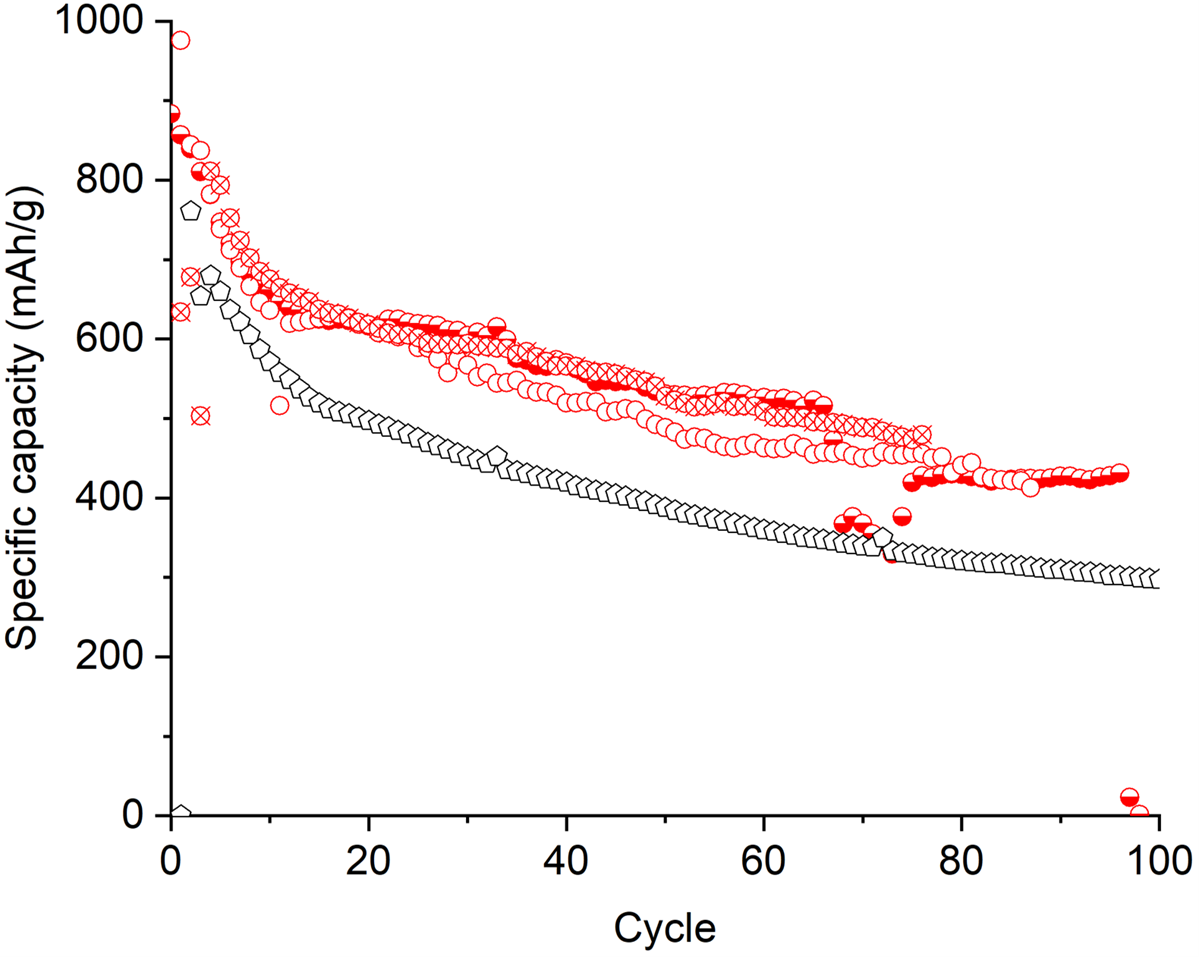
Figure 6: Specific capacity as a function of the number of charging-discharging cycles at C/10 rate for battery produced with: S10C1 composite (red dots) and reference (empty black dots) powders.
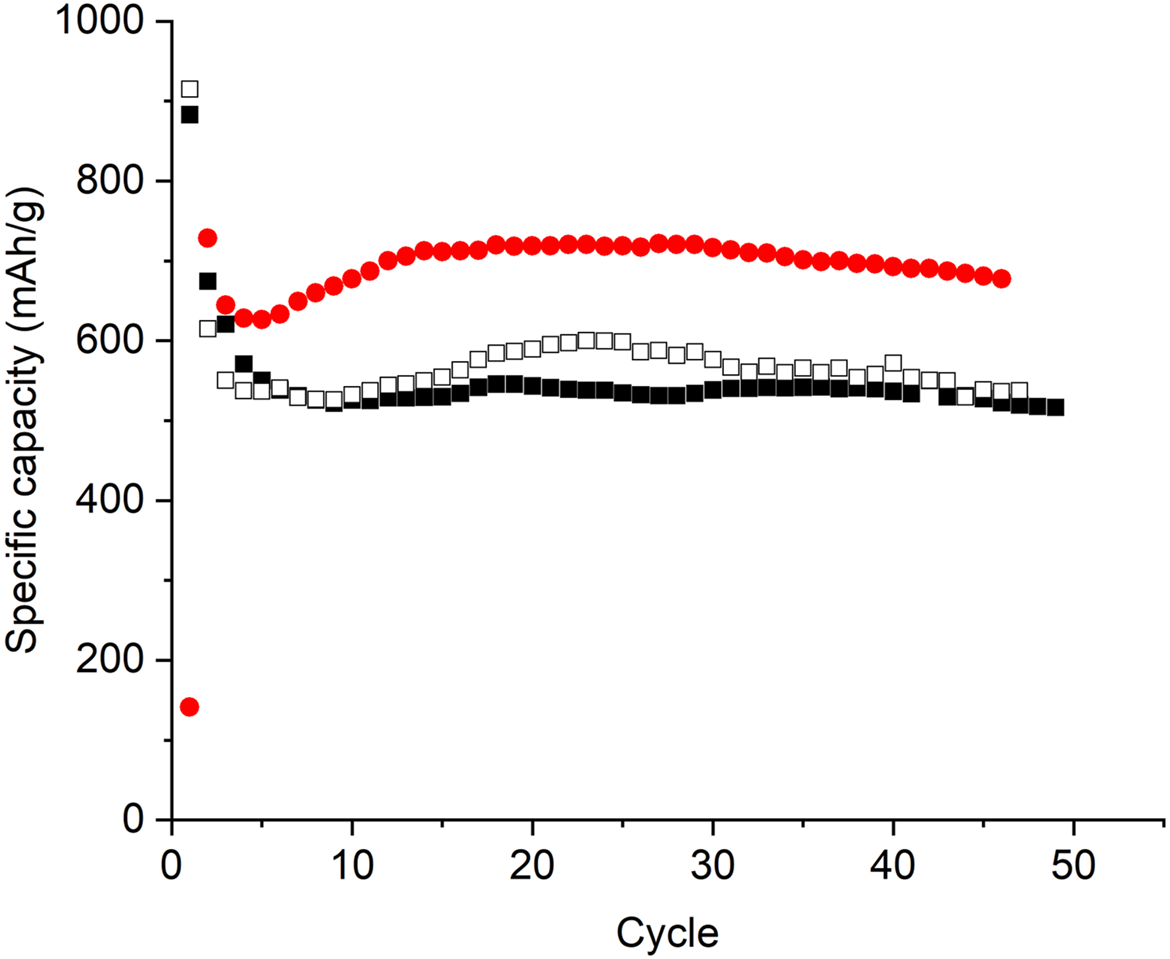
Figure 7: Specific capacity as a function of the number of charging-discharging cycles at C/40 rate for battery produced with: S10C1 composite (red dots) and reference (black and empty dots) powders.
Figure 8 presents the specific capacity at C/10 and C/40 rates as a function of the number of charging-discharging cycles for the battery produced with reference S10C2 and composite S10C2. As discussed for the S10C1 powders, composite S10C2 powder globally leads to a higher specific capacity than the reference powders at C/40 rate. However, for the C/10 rate, it was not possible to reach a high specific capacity. Indeed, the composite case shows an unstable capacity profile, contrary to the S10C1 composition.
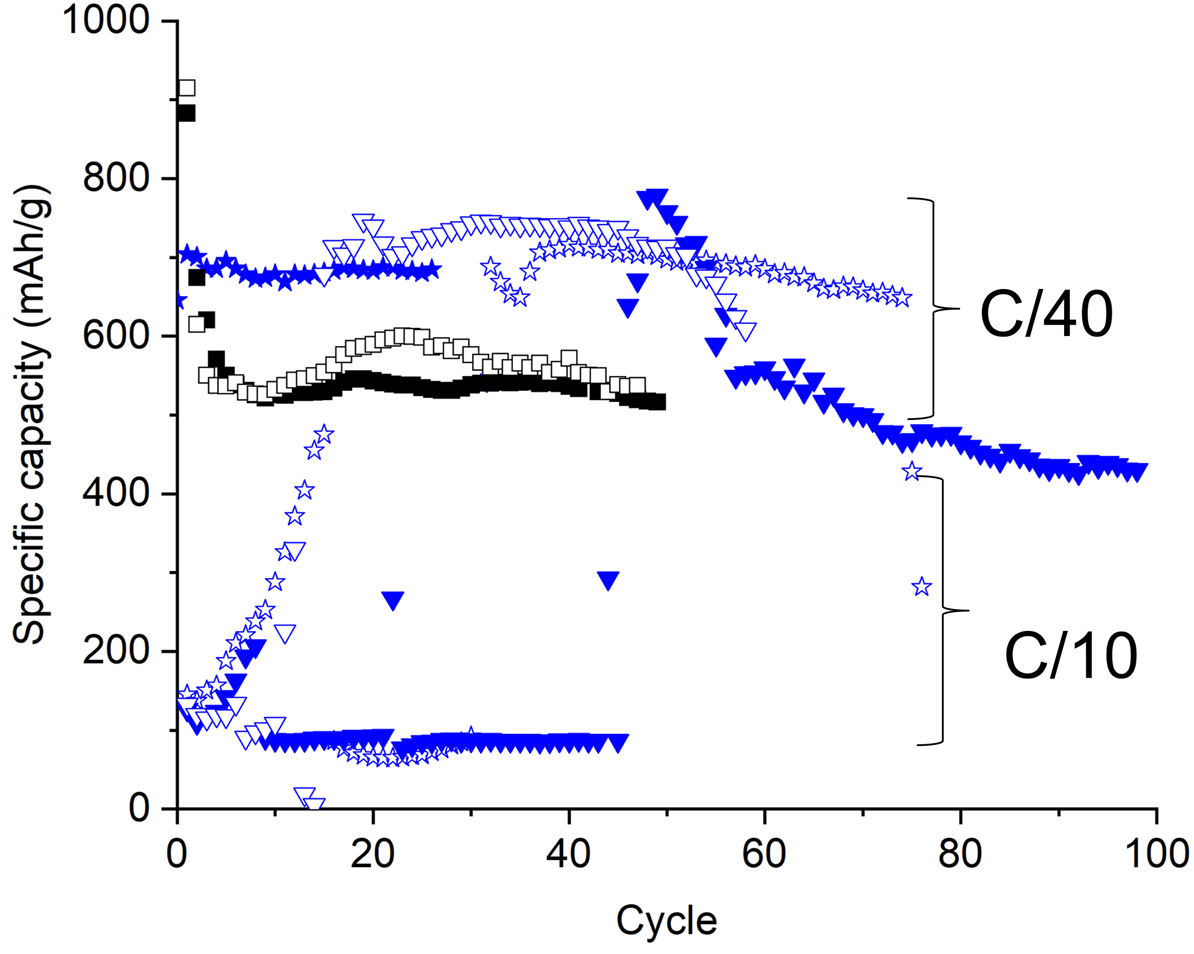
Figure 8: Specific capacity at C/10 and C/40 rates for battery produced with S10C2 composite (blue dots) and reference (black and empty dots) powders.
Discussion
Influence of production methods
The method used to prepare composite powders is observed to improve the battery performance. Indeed, for the case S10C1, the higher tapped density obtained for the composite powder corresponds to a higher specific capacity for the battery compared to what is obtained with the reference powder. These results are also corroborated to an increase of the electrode density. Indeed, the electrode surface density obtained with composite S10C1 powder was 5.00 mg/cm2 against 4.50 mg/cm2 for the electrode manufactured with the reference S10C1.
The higher tapped density observed for the composite powder compared to the reference powder increases the quantity of active material per unit of volume in the dry powder bed. If one assumes that particles integrity is preserved when composite and dry-mixed powders are blended with the binder and the rest of carbon black in a slurry to produce the electrode, the quantity of active material per unit of volume in the electrode is enhanced when produced with the composite powder, which increases the capacity of the battery.
For S10C2, the increase in specific capacity is only observed for C/40 rate. This increase in specific capacity seems also related to an increase in tapped density correlated with the increase of the final electrode surface density. For the case S10C2, 7.95 mg/cm2 was obtained for the composite against 4.50 mg/cm2 for the electrode manufactured with the reference. For C/10 rate, this link is difficult to establish due to the large variability of the capacity profile of the composite curve.
The increase in bulk density of the composite powders compared to the reference powders is clearly highlighted by the GranuPack measurement and seems induce an increase in specific capacity of the produced batteries due to the enhancement of the active material density.
Regarding the powder flowability, the reference powders are less cohesive than the composite powders. Both reference powders exhibit a shear-thinning while the S10C1 composite powder shows a shear-thickening and the S10C2 a constant cohesion. These different behaviours can negatively impact the capacity improvement provided by the composite mixture. While reference powders are assumed to show a better flowability due to their lower cohesiveness, the energy density obtained for the battery is lower than what is obtained with composite powders. On another side, composite powders would be harder to handle during dry processes due to their higher cohesion.
The shear-thickening behaviour observed for composite S10C1 can be problematic since its cohesion increases with the applied stress. During powder handling, this can limit its flowability during dry processes, like dry twin screw finder for instance, depending on the stress undergone by the powder.
Influence of carbon proportion in composite powders
The relative proportions of sulfur and carbon in the composite powders play a role in the electrode structure and the specific capacity. The battery obtained with composite S10C2 shows fluctuating results for the specific capacity, especially at C/10 rate which makes hard the comparison with composite S10C1. The large fluctuations in the capacity profile of the composite S10C2 are attributed to poor electronic percolation or heterogeneities in the electrode.
It should be noted that the electrode made with composite S10C2 has a larger surface density than the electrode made with composite S10C1, repesctively 7.95 mg/cm2 against 5.00 mg/cm while the tapped density of the composite S10C1 powder is larger than for the composite S10C2 powder. It can be hard to relate these behaviours with the tapped density of the composite powders. Indeed, the different sulfur/carbon proportions in the composite powders lead to different composite powder proportions, 72.6% for S10C1 against 79.2% for S10C2, in the slurry mixture (composite + carbon + binder) during the electrode manufacture. These differences in proportion may result in different particle organizations in the electrode structure independently of the density of the composite itself. Moreover, particle reorganization and segregation may occur during the drying of the slurry and could depend on the relative proportion of the composite powder. It could result in different electrode densities that can be hard to predict from the knowledge of the composite powder density.
The powder rheology seems also affected by the proportion of carbon in the composite powders. Further investigations should be conducted to better understand the effect of the carbon proportion on the specific capacity, the density and the rheology of the composite powders.
Conclusions
The improvement of sulfur powder for battery application is an important field of research since sulfur is a promising material to replace conventional lithium oxides for electrodes. Various processes are used to remove some drawbacks of sulfur powder and enhance its density to obtain high-capacity batteries. One of them consists of melting the sulfur and filling it into a porous medium of carbon black powder. After milling one obtains a powder whose particles are a composite of carbon and sulfur. In this work, powders prepared by this method were compared to powders prepared with a conventional dry-mixing, where the two raw powders are mechanically blended during a specific time duration at a specific intensity. The blends and composites were prepared with two proportions: 1 unit of carbon for 10 units of sulfur (S10C1) and 2 units of carbon for 10 units of sulfur (S10C2) in weight. The different powders obtained, two composite powders and two reference powders, were characterized with the GranuPack and the GranuDrum. These powders were used to produce batteries whose specific capacities were measured. From this analysis, the following main conclusions can be drawn:
- A significant increase in tapped density between dry-mixed and composite powders has been measured with the GranuPackcorrelated with an increase of electrode surface density and an increase in specific capacity of manufactured batteries.
- The packing dynamics are different between composite and dry-mixed powder, coming from differences between particle properties.
- The cohesion, measured with the GranuDrum and the Hausner ratio, of the composite powders is observed to be higher than for dry-mixed powders, probably leading to a decrease in flowability during dry processes.
- Shear-thinning was highlighted for the dry-mixed powders with the GranuDrum and shear-thickening was observed for the composite S10C1. The dry-mixed powders are then expected to have a better flowability for faster processes while the contrary could be observed for the composite powder S10C1.
Furthermore, in this application note, we highlighted the importance of suitable characterization methods to gather essential information on powder properties. Especially, the GranuDrum and the GranuPack seem adequate instruments to observe the impact of the powder preparation method on the powder behaviours. In this application note, the density improvement measured by the GranuPack for the composite preparation method is linked to an improvement of the specific capacity of the produced battery and the GranuDrum gives insight into the rheological behavours of the powders during a dry manufacturing process.
Acknowledgments
VITO (Flemish research organization in the area of cleantech and sustainable development) provided the powders and performed all the battery tests.
LEARN MORE ABOUT THE GRANUPACK
LEARN MORE ABOUT THE GRANUDRUM
Bibliography
[1] S. Li, S.Q. Zhang, L. Shen, Q. Liu, J.B. Ma, W. Lv, Y.B. He and Q.H. Yang, Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries, Adv. Sci 7, 1903088 (2020).
[2] J. Janek and W. G. Zeier, A solid future for battery development, Nat. Energy 1, 1-4 (2016).
[3] W. Zhou, Y. Li, S. Xin, J. B. Goodenough Rechargeable sodium all-solid-state battery. ACS central science. 2017 Jan 25;3(1):52-7.
[4] W. Bauer, Special features of powder technology for the production of lithium-ion batteries, Interceram-Int. Ceram. Rev. 68, 18-21 (2019).
[5] Bockholt, Henrike, et al. "The interaction of consecutive process steps in the manufacturing of lithium-ion battery electrodes with regard to structural and electrochemical properties." Journal of Power Sources 325 (2016): 140-151.
[6] H. Bockholt, W. Haselrieder and A. Kwade, Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes, Powder Technol. 297, 266-274 (2016).
[7] Nam, Young Jin, et al. "Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry-and slurry-mixing processes." Journal of Power Sources 375 (2018): 93-101.
[8] Diener, Alexander, et al. "Evaluation of Deformation Behavior and Fast Elastic Recovery of Lithium‐Ion Battery Cathodes via Direct Roll‐Gap Detection During Calendering." Energy Technology 10.4 (2022): 2101033.
